
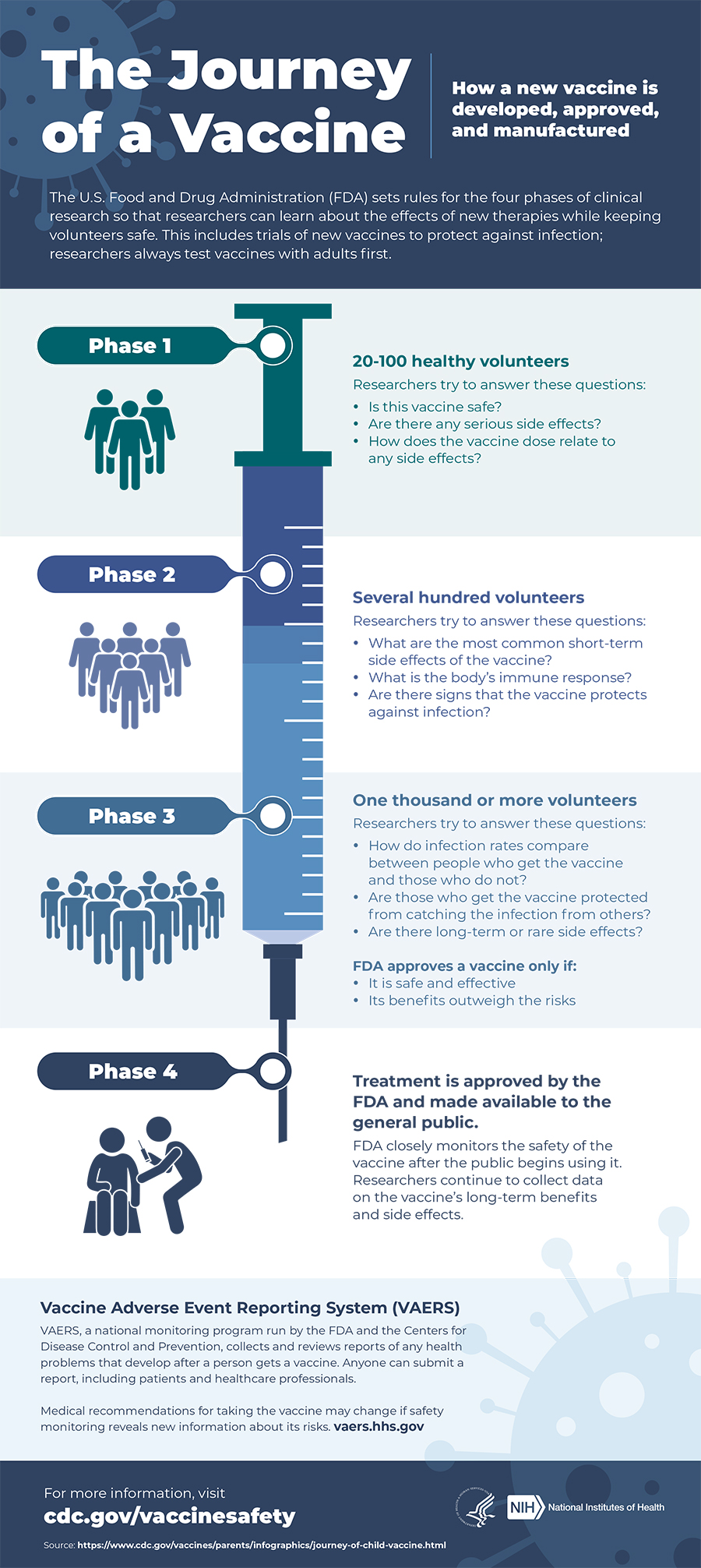
LEARN MORE ABOUT 'THE JOURNEY OF A VACCINE'
×
Frequently Asked Questions
What happens if I’m chosen to participate in the clinical trial?
You will hear from our study team to provide more information. If you are still interested, you will be invited to one of our study sites for an introductory visit, where we will provide in-depth information on the study and how it will work. You can then decide if you want to participate further.
If so, you will have appointments with clinical research staff for tests and assessments. Appointments might be at your home, at a study center, via telephone, or online.
Several tests and assessments will take place to monitor your health. These will include:
- Questionnaires about your general health and how you are feeling
- Physical examinations
- Measurement of vital signs (such as blood pressure, heart rate, breathing rate, oxygen saturation, and temperature)
- A COVID-19 test for participants
I went through the screening process but haven’t heard from anyone. Why?
Thank you for your patience with this process. There has been considerable interest in this vaccine study and our study team is working as quickly as possible to follow up with those who qualified through the pre-screening tool online.
What if I get sick during the clinical trial?
Study participants who show signs or symptoms of COVID-19 will contact the study site to begin testing for the virus and to arrange a study visit for monitoring and follow-up.
Can a vaccine give me COVID-19?
A vaccine helps our bodies develop immunity that protects the body. The vaccines under evaluation in these studies are investigational vaccines, which means that they are still in the testing and evaluation phase. The investigational vaccines won’t give you COVID-19.
Will I get the actual vaccine?
Study participants will randomly be assigned to receive either the trial vaccine or a placebo. As designed, two-thirds of participants will receive the vaccine while the remaining third will receive a placebo. To ensure the trial is fair and accurate, neither the study staff nor you will know which treatment you will receive.
Is there compensation for study participants?
Based on the number of visits and e-diaries completed, participants may receive compensation up to $1,280. There is additional reimbursement for certain expenses.
I heard on the news that vaccine trials have been stopped. Is it safe to participate now?
Vaccine trials are conducted under careful observation and review. Whenever you hear of a vaccine trial being halted temporarily, it’s due to the care taken to ensure safety for all participants. Those trials did not involve this investigational vaccine.
Who or what is Novavax?
Novavax is a U.S. biotechnology company involved in late stage development of next-generation vaccines for serious infectious diseases.